Reptation
Reptation is the snake-like thermal motion of very long linear, entangled macromolecules in polymer melts or concentrated polymer solutions.
Entanglement means the topological restriction of molecular motion by other chains.
The concept of reptation was introduced into polymer physics in 1971 by Pierre-Gilles de Gennes[1] to explain the dependence of the mobility of a macromolecule on its length. It is used as a mechanism to explain the viscous flow in an amorphous polymer.
Mechanism
Entangled polymers are characterized with effective internal scale, commonly known as ‘the length of macromolecule between adjacent entanglements’ Me.
Effective cross-links from entanglements with other polymer chains restrict polymer chain motion to a 'tube' within these restrictions. Since polymer chains would have to be broken to allow the restricted chain to pass through them, the mechanism for movement (flow) of this restricted chain is reptation. In the 'blob' model, the polymer chain is made up of 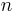 Kuhn lengths of individual length
Kuhn lengths of individual length 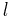 . The chain is assumed to form tangled 'blobs' between each effective cross-link, containing
. The chain is assumed to form tangled 'blobs' between each effective cross-link, containing 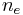 Kuhn length segments in each. The mathematics of random walks can show that the average end-to-end length of a polymer chain, made up of
Kuhn length segments in each. The mathematics of random walks can show that the average end-to-end length of a polymer chain, made up of 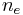 Kuhn lengths is
Kuhn lengths is 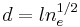 . Therefore if there are
. Therefore if there are 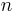 total Kuhn lengths, and
total Kuhn lengths, and 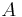 blobs on a particular chain:
blobs on a particular chain:
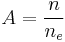
The total end-to-end length of the restricted chain 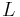 is then:
is then:
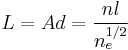
This is the average length a polymer molecule must diffuse to escape from its particular `tube', and so the characteristic time for this to happen can be calculated using diffusive equations. A classical derivation gives the reptation time 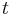 :
:
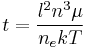
where 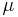 is cofficient of friction on a particular polymer chain,
is cofficient of friction on a particular polymer chain, 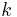 is Boltzmann's constant, and
is Boltzmann's constant, and 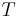 is the absolute temperature.
is the absolute temperature.
The linear macromolecules reptate, if the length of macromolecule M is bigger than ten times ‘the length of macromolecule between adjacent entanglements’ Me . There is no reptation motion for polymers with M<10 Me, so that the point 10 Me is a point of dynamic phase transition. Due to the reptation motion the coefficient of self-diffusion and conformational relaxation times of macromolecules depend on the length of macromolecule as M−2 and M3, correspondingly.[2][3] The conditions of existence of reptation in the thermal motion of macromolecules of complex architecture (macromolecules in the form of branch, star, comb and others) have not been established yet.
The dynamics of shorter chains or of long chains at short times is usually described by the Rouse model.
To be merged
Reptation theory describes the effect of polymer chain entanglements on the relationship between molecular mass and chain relaxation time (or similarly, the polymer’s zero-shear viscosity). The theory was originally developed in 1971 by Pierre-Gilles de Gennes, and has since been evolved by Sir Sam Edwards and Masao Doi. As the name suggests, the theory envisions the movement of entangled polymer chains as analogous to snakes slithering through one another. The theory predicts that, in entangled systems, the relaxation time τ is proportional to the cube of molecular mass. This is a reasonable approximation of the actual observed relationship, τ~(Molecular Mass)3.4 .
The prediction of the theory is arrived at by a relatively simple argument. First, each polymer chain is envisioned as occupying a tube of length L, through which it may move with snake-like motion (creating new sections of tube as it moves). Furthermore, if we consider a time scale comparable to τ, we may focus on the overall, global motion of the chain. Thus, we define the tube mobility as μtube=v/f , where v is the velocity of the chain when it is pulled by a force f. Note also that μtube will be inversely proportional to the degree of polymerization (and thus also inversely proportional to chain weight).
The diffusivity of the chain through the tube may then be written as Dtube=kBTμtube. By then recalling that in 1-dimension the mean square displacement due to Brownian motion is given by s(t)2 = 2Dtubet, we obtain s(t)2=2kBTμtubet. The time necessary for a polymer chain to displace the length of its original tube is then t=L2/(2kBTμtube). By noting that this time is comparable to the relaxation time , we establish that τ~L2/μtube. Since the length of the tube is proportional to the degree of polymerization, and μtube is inversely proportional to the degree of polymerization, we observe that τ~(DPn)3 (and so τ~(Molecular Mass)3).
From the preceding analysis, we see that molecular mass has a very strong effect on relaxation time in entangled polymer systems. Indeed, this is significantly different from the untangled case, where relaxation time is observed to be proportional to molecular mass. This strong effect can be understood by recognizing that, as chain length increases, the number of tangles present will dramatically increase. These tangles serve to reduce chain mobility. The corresponding increase in relaxation time can result in viscoelastic behavior, which is often observed in polymer melts.[4]
References
- ^ De Gennes P.G. Reptation of a polymer chain in the presence of fixed obstacles. J. Chem. Phys. 55, 572 - 579 (1971).
- ^ Pokrovskii V.N. A justification of the reptation-tube dynamics of a linear macromolecule in the mesoscopic approach. Physica A 366, 88-106 (2006).
- ^ Pokrovskii V.N. The reptation and diffusive modes of motion of linear macromolecules. J. Exper. Theor. Phys. 106 (3), 604-607 (2008).
- ^ Pokrovskii V.N. The mesoscopic theory of polymer dynamics. The second ed., Springer, 2010.